Twisted News item for students of bacterial cells: How transport proteins work.
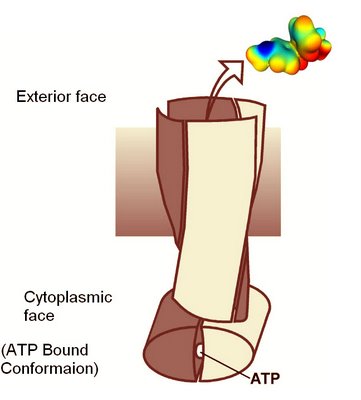
Schematic of Staphylococcus membrane transport protein in the ATP binding conformation.
A structure an ABC family transport protein that exports dyes and drugs from Staphylococcus aureus has just been reported in the journal Nature. The key discovery is that the two protein dimers in the molecule are intimately twisted around each other. The structure provides many important clues on how the transport mechanism is driven by ATP hyrdrolysis.
Structure of a bacterial multidrug ABC transporter
Roger J. P. Dawson and Kaspar P. Locher
Abstract
Multidrug transporters of the ABC family facilitate the export of diverse cytotoxic drugs across cell membranes. This is clinically relevant, as tumour cells may become resistant to agents used in chemotherapy. To understand the molecular basis of this process, we have determined the 3.0 Å crystal structure of a bacterial ABC transporter (Sav1866) from Staphylococcus aureus. The homodimeric protein consists of 12 transmembrane helices in an arrangement that is consistent with cross-linking studies and electron microscopic imaging of the human multidrug resistance protein MDR1, but critically different from that reported for the bacterial lipid flippase MsbA. The observed, outward-facing conformation reflects the ATP-bound state, with the two nucleotide-binding domains in close contact and the two transmembrane domains forming a central cavity—presumably the drug translocation pathway—that is shielded from the inner leaflet of the lipid bilayer and from the cytoplasm, but exposed to the outer leaflet and the extracellular space.
Nature advance online publication 30 August 2006 | doi:10.1038/nature05155; Received 9 May 2006; Accepted 11 August 2006; Published online 30 August 2006
Labels: membranes, physiology, transport

0 Comments:
Post a Comment
<< Home