Discovering novel antifungal agents with antimitotic activity from diverse soil and water sources.
C W S Fen, Melbourne
Introduction
Imagine if a harmless scab that you thought would just go away became the cause of your hospitalisation. Or the joy and relief of being discharged after an operation only to find yourself back in bed being treated for a post-surgical fungal infection. Fungal infections have a significant impact on the quality of life of affected individuals despite little attention dedicated to it when compared to other life-threatening diseases. However, most predominantly, it is patients who have undergone surgery, are immunocompromised, have undergone chemotherapy or excessive antibiotic doses who are severely affected and whose life may well be in danger if the infection is not addressed immediately (White et al., 1998). In view of this, biotechnological innovations and advances over the past few years can be put to good use in fuelling the initiative of discovering new or improved anti-fungal compounds which targets fungi specifically while sparing human cells.
In light of this, we propose to bring fungal-specific antimitotic drugs to a reformation, achievable by exploiting the fungal cells’ dependence on microtubule function to proliferate, and thus prevent the progression of infection. From a marketability standpoint, the diverse range of pharmaceuticals that target the ergosterol pathway in fungi are saturating the market, resulting in resistance among different drugs targeting this same biochemical pathway (White et al., 1998). Therefore the guaranteed effectiveness of this new drug minus the side effects of its competitors (which are the main source of concern), coupled with increased efficacy, is highly promising in guaranteeing a high return in investment. Accordingly, we intend to perform high-throughput screening of soil and water samples from a diverse range of sources to screen for fungi that have the ability to produce such a compound. Following that, we will run carefully designed assays to engineer additional broad-spectrum activity of the said compound. Miracles are more likely to happen if we make them and we intend on doing just that.
PROPOSAL:
The Scientific Basis
Microtubules are essential to all eukaryotic cells; in essence, fungal cell shape, transport, motility and most importantly for this proposal, cell division, rely wholly on the proper function of this critical organelle. The fundamental property of microtubules that we look to exploit in a biotechnological sense is its non-equilibrium behaviour, better known as microtubule dynamic instability, which determines the shortening or lengthening of microtubules to facilitate them in performing the functions listed (Nogales 2001). With this in mind, we analysed fungal cell characteristics to determine a logical strategy of attack.
Many secondary metabolites produced by fungi have potent pharmacological uses through exploiting its initial intended function. An obvious example of this is Penicillin produced by the mould Penicillium notatum a commonly used antibiotic for treating bacterial infections and diseases. However, this same species of mould from which penicillin is derived also produces another compound, Griseofulvin, synthesised by a close relative, Penicillium griseofulvum, which prevents microtubule formation in fungal cells during mitosis. Although griseofulvin requires a longer duration of action and exhibits less efficacy than other commonly used antifungals, nevertheless, it boasts minimal side effects and a currently still unexplored biochemical mechanism as to why it targets fungal cells specifically, partially sparing human cells and therefore resulting in milder side effects (Jimenez et al, 1990). Logically assuming that it is the specific action or higher affinity of the drug for fungi microtubules that results in the milder side effects, we propose to further expand on the merit of this particular target protein of the drug and search for novel compounds which utilises a similar mechanism of attack to halt the growth of fungi, making it a potent fungistatic agent. Subsequently (not covered by this proposal), chemical modification or further subjection of the fungi species of interest to selective chemicals or mutagenic agents can be run to develop a compound that will bind irreversibly to the microtubules and thus effectively kill off the target fungal cells (Refer to Figure 1 and Figure 2 below).
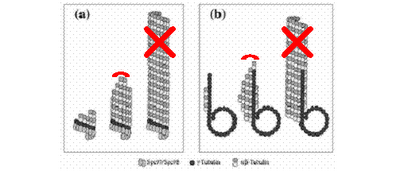 Figure 1: Two possible models of microtubule nucleation. (a) End-on model of nucleation; (b) Lateral interaction of γ-Tubulin protofilament with αβ-Tubulin dimers. (Modified from Nogales, 2001). Irreversible binding of the antifungal compound (in red – whether to the protofilament as whole or to individual dimers) can prevent prolongation of αβ-Tubulin protofilament or interaction between filaments, disrupting microtubule instability and therefore mitosis.
Figure 1: Two possible models of microtubule nucleation. (a) End-on model of nucleation; (b) Lateral interaction of γ-Tubulin protofilament with αβ-Tubulin dimers. (Modified from Nogales, 2001). Irreversible binding of the antifungal compound (in red – whether to the protofilament as whole or to individual dimers) can prevent prolongation of αβ-Tubulin protofilament or interaction between filaments, disrupting microtubule instability and therefore mitosis.
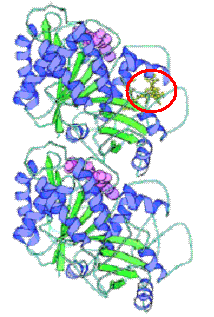 Figure 2: Ribbon diagram of molecular structure of α- and β-tubulin dimer of microtubules. A compound that can bind irreversibly to a point on this most basic unit of microtubule structure at e.g. the taxol binding site (circled in red) can trap these tubulin dimers and cause stabilization of microtubules, preventing mitosis (Revised from Nogales, 2001).
Figure 2: Ribbon diagram of molecular structure of α- and β-tubulin dimer of microtubules. A compound that can bind irreversibly to a point on this most basic unit of microtubule structure at e.g. the taxol binding site (circled in red) can trap these tubulin dimers and cause stabilization of microtubules, preventing mitosis (Revised from Nogales, 2001).
The Source
We plan to run high throughput screening of a range of soil and water samples obtained from diverse geographical locations around the world to isolate as many species of fungal cells as possible, with a more bias focus on mycoparasites, as toxic compounds produced by fungi against other fungi will be more specific and therefore more effective. Then, we will narrow down the candidates of these fungal species to those that may harbour antimitotic activities.
By diluting the soil sample in water and partially diluting the water samples, multiple cultures (100 odd petri dishes) of fungi are obtained using growth media that will stimulate fungal growth, e.g. YES media (Torres et al., 1987). After this primary culture, each fungal colony will be extracted and cultured at diluted concentrations in zone-based bioassays overlaid with a layer of one of 10 designated common infection-causing fungi, i.e. the Tinea spp., Candida spp., Aspergillus sp., Cryptococcus spp., Fusarium spp., etc. (Mays et al, 2006). It was shown by Panda et al., 2005, after a few weeks, any fungal colony whose surrounding cells are trapped in interphase and highly mononuclear exhibits antimitotic activity. These colonies are isolated and the compound produced by each colony is extracted and purified by chemical means. These compounds are then subjected to high throughput screening to test for antimitotic activity.
High Throughput Screening
The screen we have in mind is designed to be a fungal-specific antimitotic screen. Exploiting the success of genomic sequence technology and the completion of function determination of thousands of genes, we will search for genes encoding microtubule function in the 10 species of common infection-causing fungi using the available genome libraries online. By ensuring that the 10 chosen species of fungi have been studied intensively enough to for us to genetically manipulate the microtubule and its associated proteins’ genes, hypersensitive mutants susceptible to antimitotic drugs will then be generated and these mutant fungi will be used to screen for compounds that can inhibit their growth among the potential candidate compounds produced by the initial fungal culture. Testing will be done at increasing concentrations of antimitotic/antifungal compounds. Following positive results, the wild type strain for each of the 10 common disease causing fungi is then exposed to these 10 compounds as a control to identify any of the compounds can arrest the cell cycle of the parent strain (Lila et al, 2003). In this way, the antimitotic drug-sensitive fungi will increase the likelihood of discovering a drug that has antimitotic potential which we can improve the properties of through further screening.
Thus, narrowing down the search to one lead compound, we will proceed to test this in vitro with microtubules obtained from fungal cells. By tagging the fungal tubulin dimer proteins with GFP tags, we will then monitor the action of the compound on the polymerization of individual tubulin entities with increasing lead compound concentration.
From in vitro studies, we will extrapolate success of the compound in inhibiting microtubule function to in vivo testing by a reporter system where cloning technology is used to introduce fluorescence into microtubule dimers by incorporating the GFP gene into the tubulin gene (cf. protein) of each of the 10 fungal species which is then transcribed into tubulin dimers that will fluoresce, thus enabling us to monitor the growth or shrinking of microtubules (mitotic spindle) during mitosis (Implication of concept by Kumagai et al., 2003 and Alberts et al., 2002). Further proof can be obtained by running flow cytometry analysis of fungal cells treated with cytotoxic concentrations of the lead compound – if there is no increase in nuclear DNA content, then there is a lack of tubulin-related activity in vitro (Lila et al, 2003).
These same set of in vitro and in vivo experiments are repeated with human tubulin and in human cells from various tissues across the body respectively to test for toxicity of the compound (Priestly and Brown, 1978). Thus, if luck it working on our side, we may be able to determine a distinct fungal specificity of the drug and determine the exact concentration of the compound which is the threshold of toxicity for human cells. Complicated but necessary further experimentation should be done in whole organism model systems such as rats, mice and other mammals to mimic possible side effects in humans before proposing the extrapolation of these results to humans in clinical trials.
Potential Problems
Microtubules, despite being in different organisms, are highly conserved organelles, especially between organisms of the same domain (Eukaryota). We are taking a significant risk in gambling with nature on the possibility that antifungal compounds produced by fungi themselves against their fungal counterparts might specifically inhibit these competitors for survival antimitotically. At the very least however, we can design these novel compounds specifically for a distinct group of fungal species (e.g. specifically one of superficial, subcutaneous, systemic or opportunistic infection-causing groups of fungi) if not for broad-spectrum activity against most of them.
As in any microbial biotechnology process, transition from a small-scale experiment to culturing of these fungi of interest in the large scale will present us with many more problems to come. However, given the appropriate equipment, competent technicians and proficient biotechnologists, this should be a challenge we can overcome.
Further Research
As part and parcel of an antifungal treatment, side effects are inevitable as human cells are very similar structurally to fungal cells, as mentioned earlier. Thus, drugs that target fungal cells will affect our cells to a certain extent as well. Therefore, although the side effects will be specifically tailored to be minimal, further chemical modification or manipulation (if possible) to obtain compounds that have near-negligible side effects will be needed following the isolation of the fungal microtubule inhibitor protein-producing fungi..
In addition to that, also on a pharmacological basis, we need to take into account the stability of the compounds (determining optimal pH), scaling up of production and the efficiency of cell growth of the cell of interest in culture. Other than that, solubility of antifungal compounds is a major concern in the pharmaceutical industry as most antifungal compounds are contrary to that requirement. Added to that oral bioavailability, potential side effects and possible allergic reactions have to be accounted for to guarantee its economic value.
Potential Future Benefits (Integration of Science and Business)
One of the key valuable gains from the production of this initial microtubule formation inhibitor compound is a better understanding of the mechanism of microtubule inhibition and prevention of mitosis. From investigating so many fungi species with antimitotic activity, we can gain an insight into the many potential ways the microtubule can be inhibited and by manipulating this knowledge as well as other biotechnological screening and assay methods to slightly modify the compound, we could extrapolate the research to involve discovery of similar compounds from fungi which will specifically inhibit microtubule formation of tumour cells and thus secure a spin-off opportunity to produce a potential anti-cancer drug. Much research efforts and funds have been poured into a similar initiative involving Griseofulvin (Panda et al., 2005). Thus, this presents a further bonus for investing in this research.
Conclusion
In conclusion, this initiative of discovering novel antimitotic antifungal drugs presents many commercial, scientific and medical benefits to every player: investors, consumers and the biotechnologists. Besides solving a demanding medical problem and gaining much knowledge about fungi and its infection-causing mechanisms, there is much to gain from investment in the long run, with spin-off opportunities that are becoming a reality in the anticancer drug industry. “Fortune favours the prepared mind - Louis Pasteur.” This statement could not hold truer in this fascinating hybrid between business and science.
References
1. Lila, T., Renau, T.E., Wilson, L., Philips, J., Natsoulis, G., Cope, M.J., Watkins, W.J. and Buysse, J. 2003. Molecular Basis for Fungal Selectivity of Novel Antimitotic Compounds. Antimicrobial Agents and Chemotherapy. 47: 2273-2282.
2. Nogales, E. 2001. Structural Insights into Microtubule Function. Annual Review of Biophysics and Biomolecular Structure. 30:397-420.
3. Kumagai, F., Nagata, T., Yahara, N., Moriyama, Y., Horio, T., Naoi, K., Hashimoto, T., Murata, T. and Hasezawa, S. 2003. Gamma-tubulin distribution during cortical microtubule reorganization at the M/G1 interface in tobacco BY-2 cells. European Journal of Cell Biology. 82:43-51.
4. Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K. and Walter, P. 2002. Molecular Biology of The Cell, 4th edition. Garland Science, New York, USA.
5. White, T.C., Marr, K.A. and Bowden, R.A. 1998. Clinical, Cellular, and Molecular Factors That Contribute to Antifungal Drug Resistance. Clinical Microbiology Review. 11: 382-402.
6. Torres, M., Canela, R., Riba, M. and Sanchis, V. 1987. Production of patulin and griseofulvin by a strain of Penicillium griseofulvum in three different media. Mycopathologia. 99: 85-89.
7. Mays, S.R., Bogle, M.A. and Bodey, G.P. 2006. Cutaneous Fungal Infections in the Oncology Patient: Recognition and Management. American Journal of Clinical Dermatology. 7: 31-43.
8. Priestly, G.C. and Brown, J.C. 1978. Effects of griseofulvin on the morphology, growth and metabolism of fibroblasts in culture. British Journal of Dermatology. 99: 245.
Abstract
In response to the side effects and increasing amount of resistance met by antifungal drugs targeting the ergosterol pathway in fungi, we propose to bring antimitotic antifungal agents to a reformation, enlisting the use of high throughput screening to discover novel microtubule-inhibiting compounds produced by fungi from a diverse range of soil and water sources. We intend to screen large amounts of secondary metabolites produced by these fungi against 10 specifically chosen common infection-causing fungi species. After narrowing down the candidate compounds to a few lead compounds, these will be tested for fungal-specific antimitotic activity in vitro with fungal microtubule dimers and in vivo against the 10 fungi species utilizing GFP-labelling technology. Toxicity to human cells will be assessed as well before clinical trials can be proposed. Further experiments are necessary to determine optimum dosage and method of delivery of the drug, and further research into potential anticancer drug applications may yield additional commercial benefit from investment in this initiative.
Introduction
Imagine if a harmless scab that you thought would just go away became the cause of your hospitalisation. Or the joy and relief of being discharged after an operation only to find yourself back in bed being treated for a post-surgical fungal infection. Fungal infections have a significant impact on the quality of life of affected individuals despite little attention dedicated to it when compared to other life-threatening diseases. However, most predominantly, it is patients who have undergone surgery, are immunocompromised, have undergone chemotherapy or excessive antibiotic doses who are severely affected and whose life may well be in danger if the infection is not addressed immediately (White et al., 1998). In view of this, biotechnological innovations and advances over the past few years can be put to good use in fuelling the initiative of discovering new or improved anti-fungal compounds which targets fungi specifically while sparing human cells.
In light of this, we propose to bring fungal-specific antimitotic drugs to a reformation, achievable by exploiting the fungal cells’ dependence on microtubule function to proliferate, and thus prevent the progression of infection. From a marketability standpoint, the diverse range of pharmaceuticals that target the ergosterol pathway in fungi are saturating the market, resulting in resistance among different drugs targeting this same biochemical pathway (White et al., 1998). Therefore the guaranteed effectiveness of this new drug minus the side effects of its competitors (which are the main source of concern), coupled with increased efficacy, is highly promising in guaranteeing a high return in investment. Accordingly, we intend to perform high-throughput screening of soil and water samples from a diverse range of sources to screen for fungi that have the ability to produce such a compound. Following that, we will run carefully designed assays to engineer additional broad-spectrum activity of the said compound. Miracles are more likely to happen if we make them and we intend on doing just that.
PROPOSAL:
The Scientific Basis
Microtubules are essential to all eukaryotic cells; in essence, fungal cell shape, transport, motility and most importantly for this proposal, cell division, rely wholly on the proper function of this critical organelle. The fundamental property of microtubules that we look to exploit in a biotechnological sense is its non-equilibrium behaviour, better known as microtubule dynamic instability, which determines the shortening or lengthening of microtubules to facilitate them in performing the functions listed (Nogales 2001). With this in mind, we analysed fungal cell characteristics to determine a logical strategy of attack.
Many secondary metabolites produced by fungi have potent pharmacological uses through exploiting its initial intended function. An obvious example of this is Penicillin produced by the mould Penicillium notatum a commonly used antibiotic for treating bacterial infections and diseases. However, this same species of mould from which penicillin is derived also produces another compound, Griseofulvin, synthesised by a close relative, Penicillium griseofulvum, which prevents microtubule formation in fungal cells during mitosis. Although griseofulvin requires a longer duration of action and exhibits less efficacy than other commonly used antifungals, nevertheless, it boasts minimal side effects and a currently still unexplored biochemical mechanism as to why it targets fungal cells specifically, partially sparing human cells and therefore resulting in milder side effects (Jimenez et al, 1990). Logically assuming that it is the specific action or higher affinity of the drug for fungi microtubules that results in the milder side effects, we propose to further expand on the merit of this particular target protein of the drug and search for novel compounds which utilises a similar mechanism of attack to halt the growth of fungi, making it a potent fungistatic agent. Subsequently (not covered by this proposal), chemical modification or further subjection of the fungi species of interest to selective chemicals or mutagenic agents can be run to develop a compound that will bind irreversibly to the microtubules and thus effectively kill off the target fungal cells (Refer to Figure 1 and Figure 2 below).
 Figure 1: Two possible models of microtubule nucleation. (a) End-on model of nucleation; (b) Lateral interaction of γ-Tubulin protofilament with αβ-Tubulin dimers. (Modified from Nogales, 2001). Irreversible binding of the antifungal compound (in red – whether to the protofilament as whole or to individual dimers) can prevent prolongation of αβ-Tubulin protofilament or interaction between filaments, disrupting microtubule instability and therefore mitosis.
Figure 1: Two possible models of microtubule nucleation. (a) End-on model of nucleation; (b) Lateral interaction of γ-Tubulin protofilament with αβ-Tubulin dimers. (Modified from Nogales, 2001). Irreversible binding of the antifungal compound (in red – whether to the protofilament as whole or to individual dimers) can prevent prolongation of αβ-Tubulin protofilament or interaction between filaments, disrupting microtubule instability and therefore mitosis. Figure 2: Ribbon diagram of molecular structure of α- and β-tubulin dimer of microtubules. A compound that can bind irreversibly to a point on this most basic unit of microtubule structure at e.g. the taxol binding site (circled in red) can trap these tubulin dimers and cause stabilization of microtubules, preventing mitosis (Revised from Nogales, 2001).
Figure 2: Ribbon diagram of molecular structure of α- and β-tubulin dimer of microtubules. A compound that can bind irreversibly to a point on this most basic unit of microtubule structure at e.g. the taxol binding site (circled in red) can trap these tubulin dimers and cause stabilization of microtubules, preventing mitosis (Revised from Nogales, 2001).The Source
We plan to run high throughput screening of a range of soil and water samples obtained from diverse geographical locations around the world to isolate as many species of fungal cells as possible, with a more bias focus on mycoparasites, as toxic compounds produced by fungi against other fungi will be more specific and therefore more effective. Then, we will narrow down the candidates of these fungal species to those that may harbour antimitotic activities.
By diluting the soil sample in water and partially diluting the water samples, multiple cultures (100 odd petri dishes) of fungi are obtained using growth media that will stimulate fungal growth, e.g. YES media (Torres et al., 1987). After this primary culture, each fungal colony will be extracted and cultured at diluted concentrations in zone-based bioassays overlaid with a layer of one of 10 designated common infection-causing fungi, i.e. the Tinea spp., Candida spp., Aspergillus sp., Cryptococcus spp., Fusarium spp., etc. (Mays et al, 2006). It was shown by Panda et al., 2005, after a few weeks, any fungal colony whose surrounding cells are trapped in interphase and highly mononuclear exhibits antimitotic activity. These colonies are isolated and the compound produced by each colony is extracted and purified by chemical means. These compounds are then subjected to high throughput screening to test for antimitotic activity.
High Throughput Screening
The screen we have in mind is designed to be a fungal-specific antimitotic screen. Exploiting the success of genomic sequence technology and the completion of function determination of thousands of genes, we will search for genes encoding microtubule function in the 10 species of common infection-causing fungi using the available genome libraries online. By ensuring that the 10 chosen species of fungi have been studied intensively enough to for us to genetically manipulate the microtubule and its associated proteins’ genes, hypersensitive mutants susceptible to antimitotic drugs will then be generated and these mutant fungi will be used to screen for compounds that can inhibit their growth among the potential candidate compounds produced by the initial fungal culture. Testing will be done at increasing concentrations of antimitotic/antifungal compounds. Following positive results, the wild type strain for each of the 10 common disease causing fungi is then exposed to these 10 compounds as a control to identify any of the compounds can arrest the cell cycle of the parent strain (Lila et al, 2003). In this way, the antimitotic drug-sensitive fungi will increase the likelihood of discovering a drug that has antimitotic potential which we can improve the properties of through further screening.
Thus, narrowing down the search to one lead compound, we will proceed to test this in vitro with microtubules obtained from fungal cells. By tagging the fungal tubulin dimer proteins with GFP tags, we will then monitor the action of the compound on the polymerization of individual tubulin entities with increasing lead compound concentration.
From in vitro studies, we will extrapolate success of the compound in inhibiting microtubule function to in vivo testing by a reporter system where cloning technology is used to introduce fluorescence into microtubule dimers by incorporating the GFP gene into the tubulin gene (cf. protein) of each of the 10 fungal species which is then transcribed into tubulin dimers that will fluoresce, thus enabling us to monitor the growth or shrinking of microtubules (mitotic spindle) during mitosis (Implication of concept by Kumagai et al., 2003 and Alberts et al., 2002). Further proof can be obtained by running flow cytometry analysis of fungal cells treated with cytotoxic concentrations of the lead compound – if there is no increase in nuclear DNA content, then there is a lack of tubulin-related activity in vitro (Lila et al, 2003).
These same set of in vitro and in vivo experiments are repeated with human tubulin and in human cells from various tissues across the body respectively to test for toxicity of the compound (Priestly and Brown, 1978). Thus, if luck it working on our side, we may be able to determine a distinct fungal specificity of the drug and determine the exact concentration of the compound which is the threshold of toxicity for human cells. Complicated but necessary further experimentation should be done in whole organism model systems such as rats, mice and other mammals to mimic possible side effects in humans before proposing the extrapolation of these results to humans in clinical trials.
Potential Problems
Microtubules, despite being in different organisms, are highly conserved organelles, especially between organisms of the same domain (Eukaryota). We are taking a significant risk in gambling with nature on the possibility that antifungal compounds produced by fungi themselves against their fungal counterparts might specifically inhibit these competitors for survival antimitotically. At the very least however, we can design these novel compounds specifically for a distinct group of fungal species (e.g. specifically one of superficial, subcutaneous, systemic or opportunistic infection-causing groups of fungi) if not for broad-spectrum activity against most of them.
As in any microbial biotechnology process, transition from a small-scale experiment to culturing of these fungi of interest in the large scale will present us with many more problems to come. However, given the appropriate equipment, competent technicians and proficient biotechnologists, this should be a challenge we can overcome.
Further Research
As part and parcel of an antifungal treatment, side effects are inevitable as human cells are very similar structurally to fungal cells, as mentioned earlier. Thus, drugs that target fungal cells will affect our cells to a certain extent as well. Therefore, although the side effects will be specifically tailored to be minimal, further chemical modification or manipulation (if possible) to obtain compounds that have near-negligible side effects will be needed following the isolation of the fungal microtubule inhibitor protein-producing fungi..
In addition to that, also on a pharmacological basis, we need to take into account the stability of the compounds (determining optimal pH), scaling up of production and the efficiency of cell growth of the cell of interest in culture. Other than that, solubility of antifungal compounds is a major concern in the pharmaceutical industry as most antifungal compounds are contrary to that requirement. Added to that oral bioavailability, potential side effects and possible allergic reactions have to be accounted for to guarantee its economic value.
Potential Future Benefits (Integration of Science and Business)
One of the key valuable gains from the production of this initial microtubule formation inhibitor compound is a better understanding of the mechanism of microtubule inhibition and prevention of mitosis. From investigating so many fungi species with antimitotic activity, we can gain an insight into the many potential ways the microtubule can be inhibited and by manipulating this knowledge as well as other biotechnological screening and assay methods to slightly modify the compound, we could extrapolate the research to involve discovery of similar compounds from fungi which will specifically inhibit microtubule formation of tumour cells and thus secure a spin-off opportunity to produce a potential anti-cancer drug. Much research efforts and funds have been poured into a similar initiative involving Griseofulvin (Panda et al., 2005). Thus, this presents a further bonus for investing in this research.
Conclusion
In conclusion, this initiative of discovering novel antimitotic antifungal drugs presents many commercial, scientific and medical benefits to every player: investors, consumers and the biotechnologists. Besides solving a demanding medical problem and gaining much knowledge about fungi and its infection-causing mechanisms, there is much to gain from investment in the long run, with spin-off opportunities that are becoming a reality in the anticancer drug industry. “Fortune favours the prepared mind - Louis Pasteur.” This statement could not hold truer in this fascinating hybrid between business and science.
References
1. Lila, T., Renau, T.E., Wilson, L., Philips, J., Natsoulis, G., Cope, M.J., Watkins, W.J. and Buysse, J. 2003. Molecular Basis for Fungal Selectivity of Novel Antimitotic Compounds. Antimicrobial Agents and Chemotherapy. 47: 2273-2282.
2. Nogales, E. 2001. Structural Insights into Microtubule Function. Annual Review of Biophysics and Biomolecular Structure. 30:397-420.
3. Kumagai, F., Nagata, T., Yahara, N., Moriyama, Y., Horio, T., Naoi, K., Hashimoto, T., Murata, T. and Hasezawa, S. 2003. Gamma-tubulin distribution during cortical microtubule reorganization at the M/G1 interface in tobacco BY-2 cells. European Journal of Cell Biology. 82:43-51.
4. Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K. and Walter, P. 2002. Molecular Biology of The Cell, 4th edition. Garland Science, New York, USA.
5. White, T.C., Marr, K.A. and Bowden, R.A. 1998. Clinical, Cellular, and Molecular Factors That Contribute to Antifungal Drug Resistance. Clinical Microbiology Review. 11: 382-402.
6. Torres, M., Canela, R., Riba, M. and Sanchis, V. 1987. Production of patulin and griseofulvin by a strain of Penicillium griseofulvum in three different media. Mycopathologia. 99: 85-89.
7. Mays, S.R., Bogle, M.A. and Bodey, G.P. 2006. Cutaneous Fungal Infections in the Oncology Patient: Recognition and Management. American Journal of Clinical Dermatology. 7: 31-43.
8. Priestly, G.C. and Brown, J.C. 1978. Effects of griseofulvin on the morphology, growth and metabolism of fibroblasts in culture. British Journal of Dermatology. 99: 245.
Labels: Discovery, Innovation

1 Comments:
Econazole Sulfosalicylate is an antifungal agent applied topically or intravaginally. Econazole, an imidazole derivative, is indicated in the treatment of skin infections such as dermatophytosis, superficial candidasis, and tinea versicolor, Econazole Sulfosalicylate
Post a Comment
<< Home